Saunders & Walker specializes in filing lawsuits on behalf of people injured by flawed medical devices. We currently have a large number of medical device and drug lawsuits pending in both federal and state courts. Medical device lawsuits can be individual cases, coordination of individual cases in multidistrict litigation or medical class action lawsuits. Read through our list of current cases and open investigations. These cases cover everything from defective IVC blood filters and Essure birth control implants to hip and knee implant failures.
Jump To Topic
Current Saunders & Walker Medical Lawsuit Cases
Browse Categories: Hip Replacement Cases | IVC Filter Cases | Hernia Mesh | Other
Hip Replacement Recalls
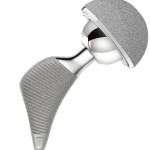
 Hip Replacement Failure, Recall, and Lawsuit
Hip Replacement Failure, Recall, and Lawsuit
Thousands of hip replacement lawsuits have been filed against manufacturers of faulty implant devices. Learn more about these suits and how victims can seek compensation for harm caused.
STRYKER LFIT V40
Another defective hip implant lawsuit is preparing to move forward. Multidistrict litigation is now underway in the U.S. District Court, District of Massachusetts involving defective Stryker LFIT V40 femoral heads.
 STRYKER REJUVENATE & STRYKER ABG II HIP IMPLANT NECK STEM DEVICE
STRYKER REJUVENATE & STRYKER ABG II HIP IMPLANT NECK STEM DEVICE
Stryker Orthopaedics has recalled two devices that are used with hip implant devices in patients undergoing hip replacement surgery. This recall comes on the heels of multiple reports of problems or adverse events related to complications or defects with both the Stryker Rejuvenate Modular Neck Stem, as well as the Stryker ABG II Modular Neck Stem. Patients have reported tissue swelling and pain related to both hip implant components. The Stryker LFIT 40 and the accolade stem failures are a part to these Stryker failures.
 ENCORE METAL ON METAL HIP IMPLANT FAILURES
ENCORE METAL ON METAL HIP IMPLANT FAILURES
The metal on metal design of the Encore hip manufactured by DJO Orthopedics has resulted in failures of the implants from metallosis requiring revision surgeries to replace the implants.
The Johnson & Johnson Depuy ASR artificial hip implant has been recalled due to adverse event reports of over 30%. The metal on metal design of these products causes a metal poisoning called metallosis that results in failure of the implants.
DePuy has paid hundreds of millions of dollars in settlements and is still paying on these cases. The DePuy Pinnacle metal on metal implant is also failing at a high rate and DePuy is still fighting those cases. Patients with these hip failures have generally had to have additional surgeries called revision surgeries to have the implant removed and replaced.
Saunders & Walker P.A. has filed a number of federal lawsuits against Biomet Orthopedics, LLC and Biomet Inc. regarding defective metal on metal Biomet M2-a Magnum hip replacement systems. There have been numerous adverse event reports involving metallosis and metal poisoning associated with this hip system.
Wright Medical Technology has had a long history of sudden failures of its profemur hip implant stems. The sudden breakage of these devices requires immediate emergency room treatment and replacement of the hip devices. Wright has had failures with both the titanium and later the chromium cobalt alloy stems.
 ZIMMER DUROM HIP IMPLANT RECALL
ZIMMER DUROM HIP IMPLANT RECALL
The implantable hip device is manufactured by Zimmer Holdings. The Zimmer hip has received complaints from surgeons and patients who state that the design is flawed, causing many patients to undergo revision hip surgery. The primary problem with the Zimmer Durom was that the cup did not properly bond with the pelvic bone causing the cups to come loose. The Zimmer Durom was also a metal on metal hip that had the metalosis failures common with that now discredited design.
 STRYKER TRIDENT HIP IMPLANT RECALL
STRYKER TRIDENT HIP IMPLANT RECALL
Stryker Corporation has issued a temporary recall of its Trident PSL and Trident Hemispherical Acetabular Cups used in hip replacement surgery. The recall was issued one week after the US FDA issued a warning letter about defects in the manufacturing processes at its Mahwah, New Jersey plant.
IVC Filter Recalls
DEFECTIVE IVC FILTERS ARE BREAKING APART
It’s estimated that about 300,000 patients have been fitted with IVC blood filter devices from Bard, Cordis, and Cook Medical over the past 30 years. Patients and their families who believe they suffered injuries from the devices are now fighting back by filing lawsuits against the manufacturer.
Hernia & Transvaginal Mesh Lawsuits
 KUGEL HERNIA MESH RECALL
KUGEL HERNIA MESH RECALL
The FDA has expanded its recall of the hernia mesh patch designed by Dr. Robert D. Kugel and manufactured by Davol, Inc. (a subsidiary of C.R. Bard). The FDA sent a letter to health care professionals and distributors on January 10, 2007, notifying them of an expansion of the recall. The lawsuits over these products have almost all been settled.
![]()
Ethicon, a division of Johnson & Johnson, removed a popular hernia repair product, Ethicon Physiomesh, from the market after it was found to cause severe side effects and surgical revisions. This mesh was recalled in May of 2016. The design of the mesh prevents tissue ingrowth and allows the mesh to move and allows reoccurrence of the hernia.
An FDA study a few years ago revealed that that transvaginal mesh implants used in female surgeries for pelvic organ prolapse (POP) and stress urinary incontinence (SUI), may cause permanent injury to women rather than benefiting them. There have been over 100,000 federal lawsuits filed against the manufacturers of these products and hundreds of millions paid in settlements. The manufacturers of TVM, tranvaginal mesh, are Ethicon, Johnson & Johnson, Boston Scientific, American Medical Systems, Bard, Covidien, and Coloplast.
OTHER
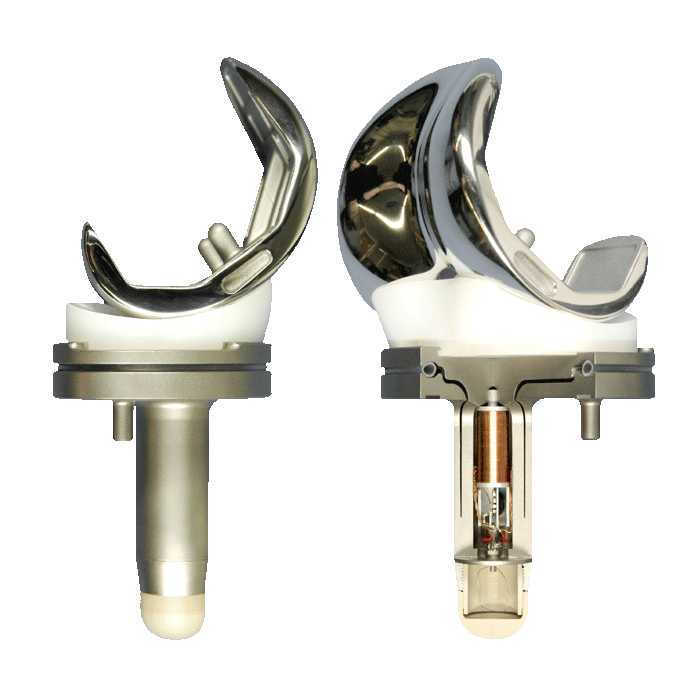 DEPUY ATTUNE KNEE IMPLANT FAILURES
DEPUY ATTUNE KNEE IMPLANT FAILURES
DePuy Attune Knee Implants have shown a high failure rate as a result of a glue failure that causes the tibial baseplate to come loose. DePuy still has not admitted the problem or issued a recall. Lawsuits in these cases are just starting to be filed. A DePuy Attune knee implant failure requires surgery to replace the device.
 BAIR HUGGER SURGICAL BODY WARMING DEVICE INFECTIONS
BAIR HUGGER SURGICAL BODY WARMING DEVICE INFECTIONS
The Bair Hugger is a forced air warming blanket system that is used in surgeries for hip and knee implants. It can contaminate the air in the operating room allowing bacteria to colonize and infect the device that is being implanted. There are hundreds of pending Bair Hugger lawsuits by patients that suffered severe surgical infections.
 SORIN MITROFLOW HEART VALVE FAILURE
SORIN MITROFLOW HEART VALVE FAILURE
Renewed attention has been focused on bioprosthetic valves manufactured by Milan-based Sorin. Previous studies indicated that the Sorin Mitroflow pericardial valve, which is used to replace damaged or diseased heart valves, had a higher than normal rate of failure. These defects have led to additional heart surgeries and death.
 ESSURE PERMANENT BIRTH CONTROL SAFETY CONCERNS
ESSURE PERMANENT BIRTH CONTROL SAFETY CONCERNS
The Essure contraceptive device has injured thousands of women since it was approved by the FDA in 2001. Over 15,000 adverse events have been reported through the FDA reporting process. FDA reported injuries include perforations of the fallopian tubes, pain, heavy bleeding, fatigue, hair loss, and even death. Many patients have needed surgery to remove the Essure device.
 ZIMMER ® M/L TAPER WITH KINECTIV® TECHNOLOGY RECALL
ZIMMER ® M/L TAPER WITH KINECTIV® TECHNOLOGY RECALL
Although only on the market for less than one month, from March 31, 2015 through April 20, 2015, the FDA announced that a Class 1 Recall is in effect as of June 8, 2015, for Zimmer’s M/L Taper with Kinectiv® Technology. A Class 1 recall is the most urgent classified by the FDA.
GranuFlo is a dry acid component of the blood cleansing mixture that is used by many commercial hemodialysis clinics. Dialysis is a process for cleaning the blood and eliminating the waste from the blood of people who have renal failure. Renal failure is the failure of the kidneys to clean the blood to eliminate waste from the blood.
 MEDTRONIC SPRINT FIDELIS DEFIBRILLATOR LEAD FAILURE
MEDTRONIC SPRINT FIDELIS DEFIBRILLATOR LEAD FAILURE
Medtronic reported that, as of October 4, 2007, there were approximately 268,000 Sprint Fidelis leads implanted worldwide, including 172,000 in the United States. Of the 268,000, about 235,000 patients still have the leads. Medtronic revealed that lead fractures may have been a possible or likely contributing factor in five patient deaths.