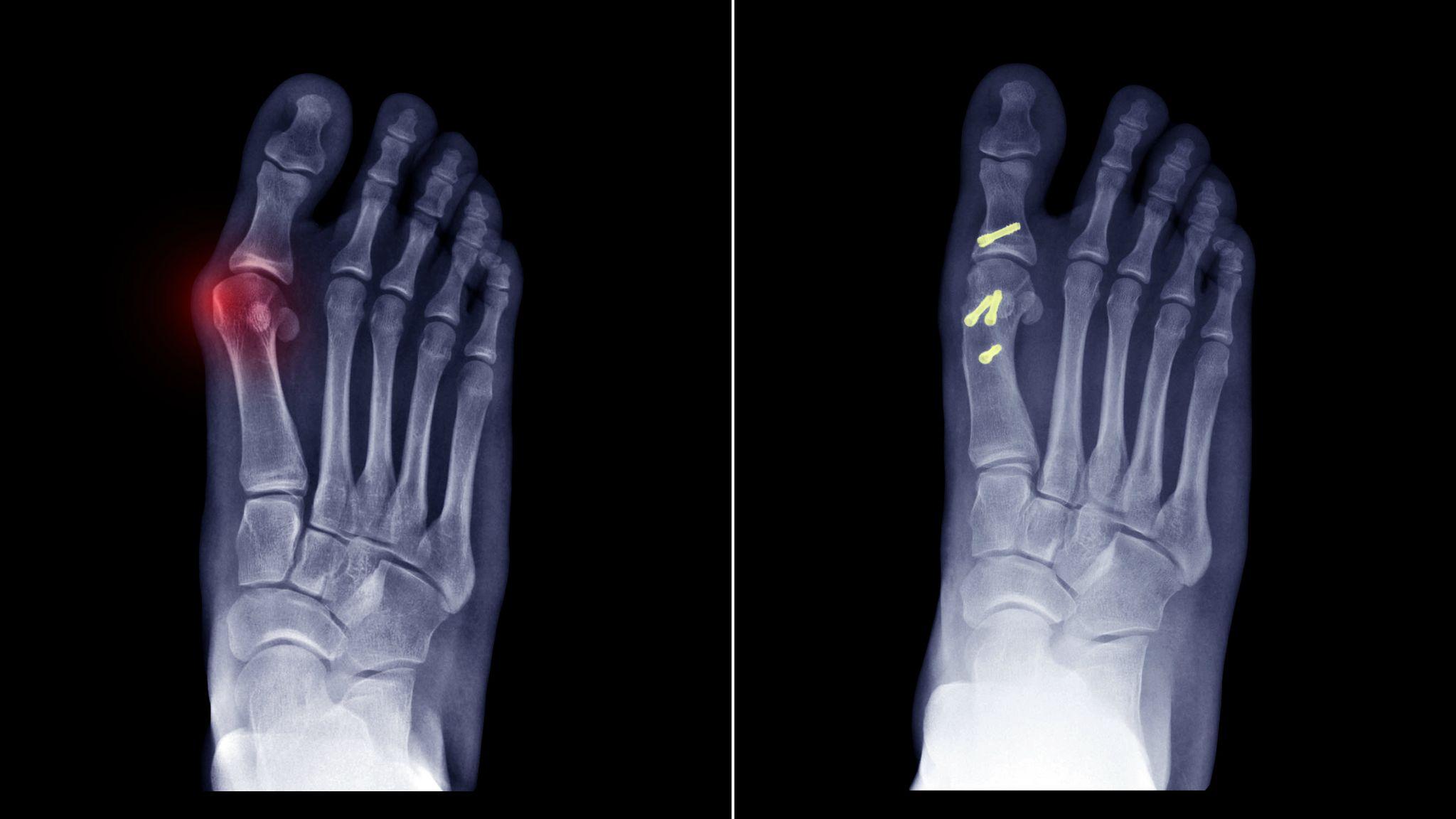
The Cartiva synthetic cartilage implant was the subject of a Class 2 FDA recall in October 2024 due to an unusually high failure rate. Many patients soon learned that instead of providing long-term relief, the implant caused worsening pain, instability, and the need for corrective surgery.
This widespread pattern of complications has led injured patients to pursue legal action, raising important questions about why the device failed, how the recall occurred, and what compensation options are available.
Let’s discuss the reasons behind the recall, the types of lawsuits being filed, symptoms of implant failure, and what your legal rights are if you were harmed by a Cartiva toe implant.
What Is a Cartiva Toe Implant?
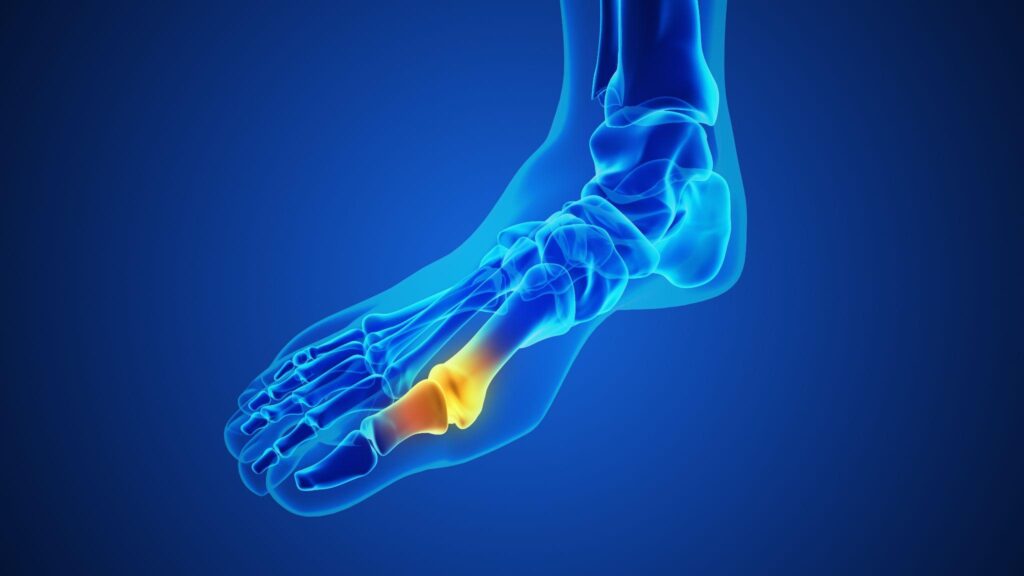
A Cartiva toe implant is a synthetic cartilage device designed to treat arthritis in the big toe joint, also known as hallux rigidus. Made from a slippery, gel-like hydrogel material, the implant is intended to mimic natural cartilage by providing cushioning and reducing painful bone-on-bone friction. Surgeons place the implant into a small cavity in the metatarsal bone to preserve joint motion while relieving stiffness and discomfort.
Although the implant was marketed as a less invasive alternative to toe fusion, many patients later experienced early implant failure, worsening pain, and the need for revision surgery—issues that ultimately contributed to its FDA recall.
Why Was the Cartiva Implant Recalled?
The Stryker Corporation, the owner of Cartiva, initiated the recall, concluding that there was an engineering design defect in the Cartiva implant that caused the failures. Internal testing and post-market surveillance revealed that the implant was breaking down far earlier than expected, leading to rapid loss of stability in the joint.
Reports from surgeons also showed a consistent pattern of the device shrinking, subsiding, or fragmenting inside the toe, which created dangerous and painful complications for patients. As these complaints increased, the FDA and Stryker determined that removing the product from the market was necessary to protect patient safety.
Symptoms of Failed Cartiva Toe Implants
Symptoms of a failed Cartiva toe implant may include:
- Persistent or worsening pain
- Implant subsidence or displacement
- Nerve damage
- Implant fragmentation or breaking apart
- Need for removal or revision surgery due to implant failure
Suing for a Defective Toe Implant

Medical device manufacturing companies that have sold implant devices that were defectively designed can be held liable for financial compensation by patients who have suffered from device failures.
Usually, the filing of a lawsuit is necessary before these companies will pay compensation for the pain, suffering, medical bills, or lost wages incurred by patients with defective implants.
Cartiva Settled Lawsuits

Cartiva has already settled some of the lawsuits that have been filed within the last few years over this failed toe implant. There will likely be a hearing in federal court in January 2026 before the federal Judicial Panel on Multi-District Litigation to consider coordinating all of the federal lawsuits that have been filed before one judge.
This process will allow lawyers for persons injured by this recalled implant to share information and experts with each other to better prepare the product liability cases for trial or settlement.
Cartiva Individual Lawsuits
The lawsuits being filed for injured implant patients are individual cases, not class action lawsuits. Class action lawsuits are generally only certified for return of the purchase price of the devices, not for the individual injuries to the persons involved.
Unlike a class action, these individual lawsuits entitle each person to make the decision about settlement or trial in their case, rather than a one-size-fits-all class action settlement.
The cases are likely to be coordinated together in a federal Multi-District Litigation for pre-trial management and discovery, but each individual retains the right guaranteed by the Seventh Amendment of the US Constitution to an individual jury trial in their case.
Representation From Saunders & Walker P.A.

Our law firm has decades of experience handling medical device lawsuits in federal and state courts throughout the United States. We are happy to review your Cartiva toe implant case at no charge and to represent you on a fee contingent on the outcome if we accept your case.
If you’re looking for a trusted defective medical device lawyer, contact Saunders & Walker P.A. today.

